Is your AI software a medical device as per the MDR & IVDR?

The advancements of technology in the healthcare industry have led to a paradigm shift in this sector. The rapid pace at which software has impacted the field have also led to regulatory responses, resulting in some softwares now being considered as medical devices.
Nevertheless, defining whether your software must comply with the Medical Device Regulation can be challenging. The EC, states that “software must have a medical purpose on its own to be qualified as a medical device software (MDSW)” on its Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR . Artificial Intelligence software in the medical field is, from now on, largely regulated in the same way as traditional software. More specifically, the EC’s Medical Device Regulation (Regulation (EU) 2017/745 of the European Parliament and of the Council of April 5, 2017 on medical devices), explicitly included Artificial Intelligence softwares in the regulation.
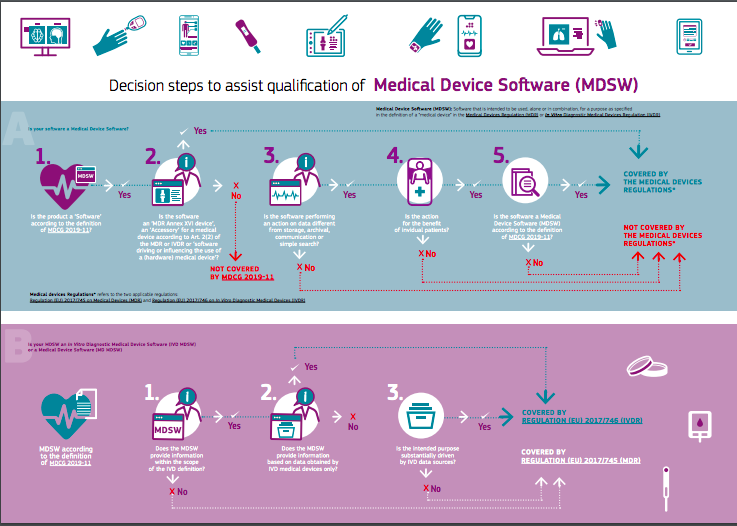
In order to facilitate the process of identifying whether or not a software is a medical device, the EC has published, as of March 2021, a visual chart that helps to define whether a software can be classified as a medical device or not.
The visual shows the steps taken to classify softwares (A) and in vitro diagnostic medical device softwares (B). As regards to softwares, they need to:
- Be classified as softwares according to the MDCG 2019-11
- Be an accessory for a medical device or a software driving or influencing the use of a hardware medical device according to the relevant regulations
- Perform different actions on data from storage, archival, communication or simple search
- The action needs to be for the benefit of the patient
- Be a medical device software in accordance to the definition of MDCG 2019-11
Some examples of softwares and their classification:
- Regular software which isn't a medical device: A software that backs up electronic medical records.
- Regular software which is a medical device: A software that triggers an alarm when a patient stops breathing.
- AI-based software which isn't a medical device: An AI algorithm that summarizes medical literature
- AI-based software which is a medical device: An AI algorithm that guides a doctor in diagnosing a certain condition.
Any company active in the healthcare sector is likely to face the question of whether all parts of the software they are developing can be impacted by this regulation, and as such, knowing the rules and their impact can influence both the feasibility and outcome of projects tremendously.
If you are considering developing an AI based software, don’t hesitate to contact us. Kantify is expert in developing AI solutions for the healthcare sector. We ensure that the tech is not only well built, but also compliant with the regulation!